Bluesky Explainer thread by Dr. Kate Kennedy here:
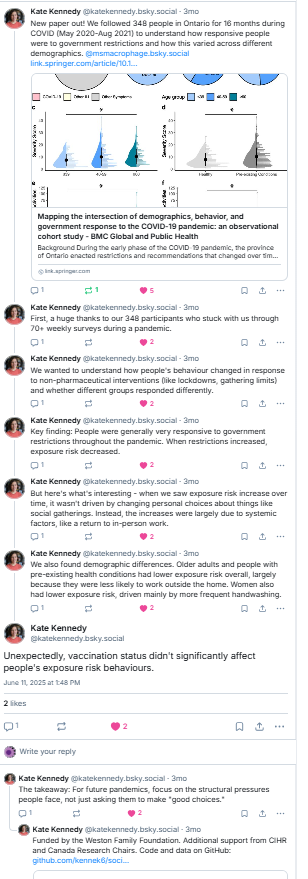

Bluesky Explainer thread by Dr. Kate Kennedy here:

Below is an ‘explainer’ thread from Bluesky. See original here
Publication alert: “No evidence of immune exhaustion after repeated SARS-CoV-2 vaccination in vulnerable and healthy populations” @natcomms.nature.com The backstory is particularly interesting-it’s a tale of the conflicting needs of scientists & decision makers in times of disinformation ….1/n
To begin – my team was funded by the CITF to study COVID-19 infections/vaccinations in older adults & people on immunosuppressants. We had a broad mandate to look at ‘cellular & humoral immunity’ and unlike most grants there was a constant feedback to decision makers, participants & the public 2/n https://www.covid19immunitytaskforce.ca/
This meant that I got a pretty good sense of people’s worries and concerns and we could get solid data to address them. Case in point – we provided data that long-term care residents needed a 3rd dose and then a 4th – they got them and colleagues proved they prevented many infections 3/n https://www.bmj.com/content/378/bmj-2022-071502.short
In 2021 internet personalities were fretting that too many vaccines would lead to ‘immune exhaustion’. Most immunologists were not worried (explanation to follow) but I was shocked to hear in meetings that some folks on decision making tables were worried, esp, for vulnerable populations 4/n
What is this scary ‘immune exhaustion’? When you turn on an inflammatory response (e.g., by vaccination/infection) you have to have a way to turn it off. T cells that recognize an antigen multiply, make cytokines and start to express ‘off-switches’ which have names like PD-1, Tim-3 & Lag-3. 5/n
When there is a lot of antigen around (ie. during infection or vaccination) the balance between the on-switch (antigen-stimulation) and the off-switch (PD-1 & friends) favours being on – the T cells expand & differentiate & work their magic. Here PD1 is better described as an ‘activation marker’….. 6/n
…than an exhaustion marker. When the antigen decreases (ie infection or vaccine clears), the off-switches signal that it’s time to close up the inflammatory shop. It is pretty rare that antigens don’t go away but in cancer that chronic stimulation can lead to sustained expression of the PD1 off-switch. 7/n
How do you know when a T cell is truly ‘exhausted’? It expressed these markers AND it loses its function. Here’s where our study shines – we looked at the T cells’ ability to produce at least 1 cytokine (‘functionality’) or more than 1 cytokine (‘polyfunctionality’). 8/n
Repeat vaccination does not affect the T cells ability to make cytokines, even in vulnerable populations. They may express some activation markers but they are definitely not turned off. 9/n
Pity the lead author, PhD student @jennabenoit.bsky.social Her committee would grill her ‘why are you doing this- we all know vaccines don’t cause exhaustion!’ She held strong “Because policymakers & the public need us to PROVE this in THESE people”-She was strong, thorough & committed. 10/n
So why do so many people make such rash statements on whether a T cell is doing it’s thing (PD-1 = activation) vs crashing out (PD-1 =exhausted)? Above I said this disinformation began circulating in 2021 – we’ve been working on this story since then 11/n
Measuring 1 marker (eg PD1) and making wild inferences is relatively cheap & quick but measuring T cell function and analyzing these complex data is slow & expensive. Indeed we had a team of people working very hard in a clinical trials quality immune testing lab and an analysis team to generate these data. 12/n
https://healthresearch.healthsci.mcmaster.ca/single-cell-spatial-profiling-core-facility/human-immune-monitoring-services/
Learnings: Disinformation = quick to make up but hard to disprove and even immunologists and experts can have seeds of doubt sown by bad actors. Vulnerable populations deserve to be included in research. Negative data studies are impt but hard to sell (see reviews of the paper!). 13/n
Huge shout out to lead author @jennabenoit.bsky.social and the not-on-Bluesky team from our star phlebotomist/blood processor Braeden Cowbrough, flow cytometry genius Dr. Jessica Breznik, analytics guru Dr. Chris Verschoor, and HITS Team (Nichols, Hagerman, Bramson) and of course our participants. 14/14
Serosurveillance describes using blood samples to determine what percentage of the population has been exposed to a pathogen or has been vaccinated by measuring the presence or absence of antibodies to the pathogen or vaccination. During the COVID-19 pandemic Dr. Bowdish and team built a network of long-term care homes to measure vaccine responses and infection rates, but this infrastructure could have been used to measure virtually any infection or any antibody response. The Hema-Net community came together February 14-16 to present data and share experiences using serosurveillance and published this report. Unfortunately, no funds were made available to continue Dr. Bowdish’s or others serosurveillance networks.
See the CITF website here.
See the English report here.
See the French Report here.
Click here to access: Monocyte-driven inflamm-aging reduces intestinal barrier function in females published in Immunity and Ageing, September 2024.
This publication by former PDF, Dr Candice Quin and team, discovers that inflammatory markers and gut permeability increase with age, but the leaky gut seems to be a female specific phenomena in both mice and humans.
Bluesky explainer thread below and here https://bsky.app/profile/msmacrophage.bsky.social/post/3l5ethyexgp2u
New publication alert! “Monocyte-driven inflamm-aging reduces
intestinal barrier function in females” by lead author Dr Candice Quin @uniofaberdeen.bsky.social. Read on for some surprising insights into sex differences in aging, the microbiome, inflammation, and the ‘leaky gut’ hypothesis….1/n
With age levels of inflammatory mediators (cytokines, CRP, & others) increase in the blood and tissues. This is often called ‘inflamm-aging’, and higher than age-average levels of these mediators are associated with chronic disease, frailty, and other age-associated ills. 2/n
Men & women age differently (‘men die quicker, women live sicker’)so it stands to reason they might ‘inflam-age’ differently, but very few studies on aging investigate sex differences in the aging trajectory. We looked at cellular & soluble inflammatory markers and saw sex differences! 3/n
We were surprised by the magnitude of sex differences but immunology is literally one of the worst disciplines when it comes to reporting by sex https://elifesciences.org/articles/70817
By why do we ‘inflamm-age’ in the first place? Might the mechanisms of inflamm-aging differ by sex 4/n
One of the major theories is that with age a dysbiotic microbiota causes the gut to become leaky (alternatively, the gut gets leaky with age and this alters the microbiota). Bacterial products leak out and cause inflammation. We’ve published in mice, others in other model organisms 5/n https://www.cell.com/cell-host-microbe/fulltext/S1931-3128(17)30112-9?elsca1=etoc&elsca2=email&elsca3=1931-3128_20170412_21_4_&elsca4=Cell%20Press
Evidence for the ‘leaky gut’ hypothesis is weak in healthy humans (despite what the probiotic industry might say). Leakiness is generally found in people who have comorbidities, or are frail so we looked at healthy/non-frail male (41) females (54) btw 20-102 yrs and were surprised to find…6/n
…that women appeared to have leakier guts over the life course (as measured by the serum marker zonulin- imperfect), and only women had evidence of an age-associated increase in the bacterial product LPS in their serum. Could the leaky gut hypothesis be female specific? 7/n
Human studies are observational so we turned to mice to understand the chain of causality. Old female, but not male, mice did indeed have leakier guts and this seemed to be caused by TNF produced by the elevated number of circulating inflammatory monocytes. 8/n
Tl;dr The age-related leaky gut -> increased circulating bacterial products -> systemic inflammation hypothesis may only be true in females! (also – always investigate sex in your research) 9/n
Why? Well the female gut changes dramatically during pregnancy to increase nutrient absorption – perhaps that is why women seem to have more permeability over the life-course. We are doing some cool ongoing studies in vitro looking at male vs female monocytes ability to break the gut barrier. 10/n
Caveats: This was a non-frail, mostly white population – aging & immunology studies show tremendous differences by location so needs to be reproduced. Gut permeability measures were done by looking at serum markers – easy on the participant but indirect at best. 11/n
Thanks to the whole (unfortunately not on Bluesky team)! This was an incredibly fun and challenging project to work on but has cemented my commitment to considering sex in all the research we do. 👩🔬🧪🦠🚨 12/12
Download PDF here.
Link to journal here.
Abstract: Somatic mutations in the TET2 gene occur more frequently with age, imparting an intrinsic hematopoietic stem cells (HSCs) advantage and contributing to a phenomenon termed clonal hematopoiesis of indeterminate potential (CHIP). Individuals with TET2-mutant CHIP have a higher risk of developing myeloid neoplasms and other aging-related conditions. Despite its role in unhealthy aging, the extrinsic mechanisms driving TET2-mutant CHIP clonal expansion remain unclear. We previously showed an environment containing tumor necrosis factor (TNF) favors TET2-mutant HSC expansion in vitro. We therefore postulated that age-related increases in TNF also provide an advantage to HSCs with TET2 mutations in vivo. To test this hypothesis, we generated mixed bone marrow chimeric mice of old wild-type (WT) and TNF–/– genotypes reconstituted with WT CD45.1+ and Tet2–/– CD45.2+ HSCs. We show that age-associated increases in TNF dramatically increased the expansion
of Tet2–/– cells in old WT recipient mice, with strong skewing toward the myeloid lineage. This aberrant myelomonocytic advantage was mitigated in old TNF–/– recipient mice, suggesting that TNF signaling is essential for the expansion Tet2-mutant myeloid clones. Examination of human patients with rheumatoid arthritis with clonal hematopoiesis revealed that hematopoietic cells carrying certain mutations, including in TET2, may be sensitive to reduced TNF bioactivity following blockade with adalimumab. This suggests that targeting TNF may reduce the burden of some forms of CHIP. To our knowledge, this is the first evidence to demonstrate that TNF has a causal role in driving TET2-mutant CHIP in vivo. These findings highlight TNF as a candidate therapeutic target to control TET2-mutant CHIP.
Bluesky explainer thread here:
New Paper Alert: “Chronic TNF in the aging microenvironment exacerbates Tet2 lost-of-function myeloid expansion” published in Blood Advances #ImmunoSky #CHIP https://authors.elsevier.com/sd/article/S2473952924003860 (1/n)
Over time the progenitor cells (#stemcells) in our bone marrow acquire random mutations, with some genes being more likely to acquire these mutations than other. The Tet2 gene is one that acquires mutations. This is a problem because it regulates other genes through methylation (2/n)
Hematopoetic #stemcells with Tet2 mutations favour production of myeloid cells (#neutrophils #monocytes) over lymphoid cells (#Tcells #Bcells) and can outcompete stem cells that don’t have Tet2 mutations.(3/n)
If too many white blood cells originate from these mutants it is called Clonal Hematopoesis of Indeterminate Potential or #CHIP. CHIP is associated with an increase in mortality, and pneumonia (see prev paper), because immune cells with these mutations don’t work as well (4/n) https://shorturl.at/JNk6d
With age the proportion of Tet2 mutant cells increases and it is thought that the increased #inflammation that occurs with age contributes. Darwinian evolution happens in the bone marrow and stem cells with Tet2 mutations can outcompete (i.e. replicate more, make more white blood cells) than those without (5/n)
We investigated whether Tet2 mutant stem cells could outcompete others in the presence of the inflammatory cytokine TNF, which increases with age. We put a mix of normal and Tet2-/- stem cells into old mice that had lots of TNF and those with none (TNF KO). 6/n
Stem cells with Tet2 mutations could outcompete normal cells in old mice that had lots of TNF but not in TNF knockout mice. The aging inflammatory microenvironment contributes to CHIP! (7/n)
Cool finding- People with newly diagnosed #rheumatoidarthritis have lots of TNF and had low level of Tet2 and other CHIP mutants that went down as they started anti-TNF or anti-inflammatory therapy! Proof this happens in humans too (8/n)
Huge thank you to not on Bluesky Drs Michael Rauh (Queens), Candice Quin ( @uniofaberdeen.bsky.social ), Maggie Larche (UCalgary), Salman Basrai&Sagi Abelson @oicr.bsky.social and team. (9/9)
Jenna Benoit (PhD candidate) has published her first, first author paper characterizing how immune responses to vaccination differ in people living with rheumatoid arthritis. We found some interesting new drug-immune interactions.
See thread here: https://bsky.app/profile/msmacrophage.bsky.social/post/3kh2uswvqtm2u
or below….
New paper alert! @jennabenoit.bsky.social and team studied COVID-19 vaccinations in people living with rheumatoid arthritis who are on immunosuppressive drugs and found some interesting, and to our knowledge, unknown effects of specific drugs 1/n
Almost all studies of vaccine immunogenicity (i.e., how strong an immune response is to a vaccine) focus on antibody responses. Measuring the amount of antibodies produced is cheap and (relatively) easy; however, in the Omicron-era these are less predictive of protection than you might think 2/n
When investigating anti-receptor binding domain (RBD) antibodies @jennabenoit.bsky.social and team found that -unsurprisingly- people living with RA and men had lower antibody responses (men have lower antibody responses to vaccination in general), and people with COVID had higher responses (i.e., that hybrid immunity you’ve heard so much about) 3/n
What caused these lower antibody responses? DMARDs (disease modifying anti-rheumatic drugs),and anti-TNF were not associated with lower antibody levels, the effect of steroids was not significant, but costimulation inhibitors reduced antibody levels 4/n
Important caveat: The effect of co-stimulation inhibitors was about the same as being a biologic male, so whether this reduction is associated with increased risk of infection or not is not something we can comment on 5/n.
We didn’t see an effect of drugs on neutralizing antibodies (i.e., antibodies that bind the virus really well and prevent it from entering us), but we did not have enough people on some of the drugs to really investigate this 6/n.
My favourite part: CD4+ and CD8+ T cell responses to vaccination are much, much harder to measure (each dot on the graph costs about $350 and 3+ hrs of time – hence the ‘team’ I keep mentioning) but we know that they are important for preventing infection.7/n
We found that people living with RA had lower CD4+ T cell responses (= ‘helper’ cells that support many aspects of the immune response to infection & vaccination), those who had had COVID were higher – more of that hybrid immunity you’ve heard about. 8/n
BUT even though we had a small number of people on JAK inhibitors, those who were on them had markedly lower CD4+ responses. The effect of co-stimulation inhibitors was not as apparent – but again low numbers of participants so hard to say. 9/n
Speculative side note: We use influenza vaccine as a control. Everyone has had exposure as kids so this measures a memory response made prior to having been vaccinated. Co-stim inhibitors don’t affect influenza but JAK inhibitors do – therefore no defect in pre-drug immune responses? 10/n
CD8+ T cell responses (‘killers’ of virus infected cells), were higher in men (previously known), and didn’t seem to be lower in most drugs, except maybe steroids. 11/n
Caveats: Our study was small and due to the fact we were measuring 1,2,3 doses, we were recruiting fast and furious and didn’t capture as many people on some of the drugs as we would have liked,so all results need to be replicated. 12/n
Clinical relevance: Some of these drugs are associated with increased risk of severe disease (see text for references) and by learning which aspects of the immune response they affect, we learn which aspects of the immune response are required for a successful vaccine. 13/n
Deepest appreciation for our research participants, the Canadian Arthritis Patient Alliance (see website for talks on this topic), the SUCCEED investigator team, our technical staff, fundign from the Public Health Agency of Canada, and you for reading to 14/n
Dr. Dawn Bowdish and her PhD student Dessi Loukov collaborated with Dr. Monica Maly and Sara Karampatos (Rehabilitation Science) and found that monocytes were more activated and pro-inflammatory in women with osteoarthritis, and that elevated inflammation and body mass index were associated with increased monocyte activation. Further, the team found that women with osteoarthritis and more activated monocytes experienced worse pain than individuals with less activated monocytes. These findings highlight the importance of modulating inflammation and body mass to manage osteoarthritis and open up new avenues for therapeutic research.
Read the full publication in the Osteoarthritis Research Society International (OARSI) Journal
As featured in Eureka Alert: https://www.eurekalert.org/pub_releases/2017-11/mu-rul112717.php
First author on the publication, PhD student Kyle Novakowski of Dr. Dawn Bowdish’s lab.
A common element that links ancient fish that dwell in the darkest depths of the oceans to land mammals, Neanderthals, and humans is the necessity to defend against pathogens. Hundreds of millions of years of evolution have shaped how our innate immune cells, such as macrophages, detect and destroy microorganisms.
In a new study led by Dr. Dawn Bowdish (in collaboration with Dr. Brian Golding) and her PhD student Kyle Novakowski, the team identified novel sites within a macrophage receptor, MARCO, that are under positive selection and are human-specific. The team demonstrated the importance of these sites by site-directed mutation and showed a reduction in cellular binding and uptake of pathogens. These findings demonstrate how small genetic changes in humans can influence how we defend ourselves against pathogens.
Read the full publication in Oxford University Press.